Written by: Gabriele Vieira; Reviewed by: Olga Kildisheva
The tetrazolium test (TZT) has been developed and refined since the early 20th century, originally designed to assess seed viability. Over time, methodological advances have expanded its application to include the evaluation of seed vigor across various species, including forest species.
Seed viability and seed vigor
Seed viability refers to the ability of a seed to remain alive and capable of germination under ideal conditions. It indicates the presence of metabolically active and structurally intact embryonic tissues capable of developing into a normal seedling. Seed vigor, on the other hand, is a broader concept that encompasses the seed’s potential to germinate rapidly, uniformly, and produce strong seedlings even under suboptimal or stressful environmental conditions. While all vigorous seeds are viable, not all viable seeds are necessarily vigorous. Both attributes are essential for successful seedling establishment and can be evaluated through physiological and biochemical tests, such as the tetrazolium test.
Principles of the test
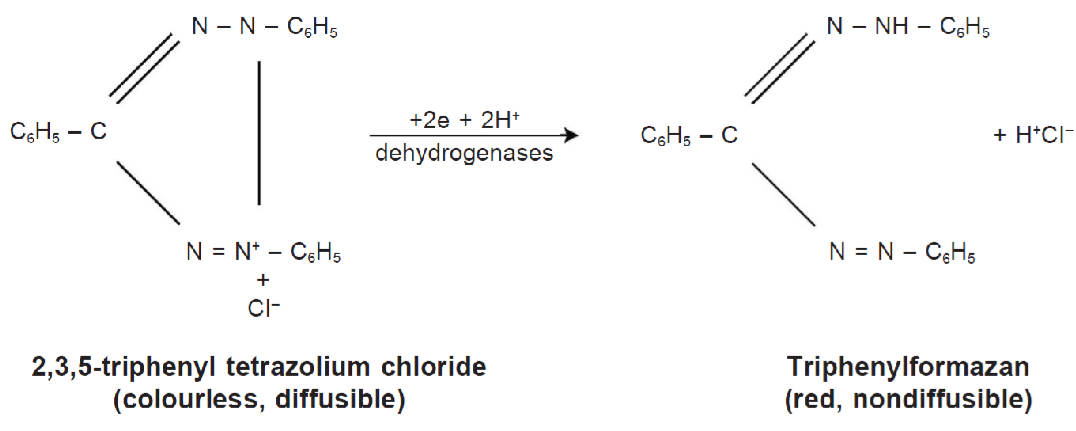
The TZT indirectly assesses the respiratory activity of the cells that make up seed tissues. It is based on the activity of dehydrogenase enzymes, which catalyze key reactions in mitochondrial respiration—particularly during glycolysis and the Krebs cycle. These enzymes reduce the colorless tetrazolium salt in metabolically active (living) tissues.
When immersed in the colorless tetrazolium solution, the dye penetrates seed tissues and interferes with the reduction processes of the living cells by accepting a hydrogen ion. In its reduced form, the tetrazolium solution becomes a red-colored, stable, non-diffusible substance called triphenyl formazan, or simply formazan.
Tetrazolium reduction reaction, Source: França-Neto, J. B.
Procedures of the test
Seeds are initially preconditioned using moist germination paper (slow moistening) to reach moisture levels that activate mitochondrial respiration. This method is preferred for larger seeds, as it allows tissues to imbibe water without damage and prevents death from anaerobic conditions like full water immersion. For smaller seeds, water immersion is a faster alternative but requires careful timing to avoid tissue damage.
Preconditioning may also include dormancy-breaking treatments. Seeds often need cutting, perforation, or seed coat removal to facilitate water and tetrazolium solution uptake. Sharp tools must be used to avoid damaging seeds and compromising test accuracy.
Tetrazolium solutions at 1% or 0.5% concentrations are common, with pH maintained between 6.5 and 7.5 to ensure proper staining. A 1% solution is prepared by dissolving 10 g of tetrazolium salt in 1 liter of distilled water.
During staining, seeds must be fully submerged and protected from light to prevent unwanted salt reduction. After staining in a light-proof container, seeds are incubated at species-specific temperatures and durations, following established protocols when available. Concentrations can vary if results remain reliable. Finally, seeds are rinsed to remove excess solution.
Tetrazolium test interpretation
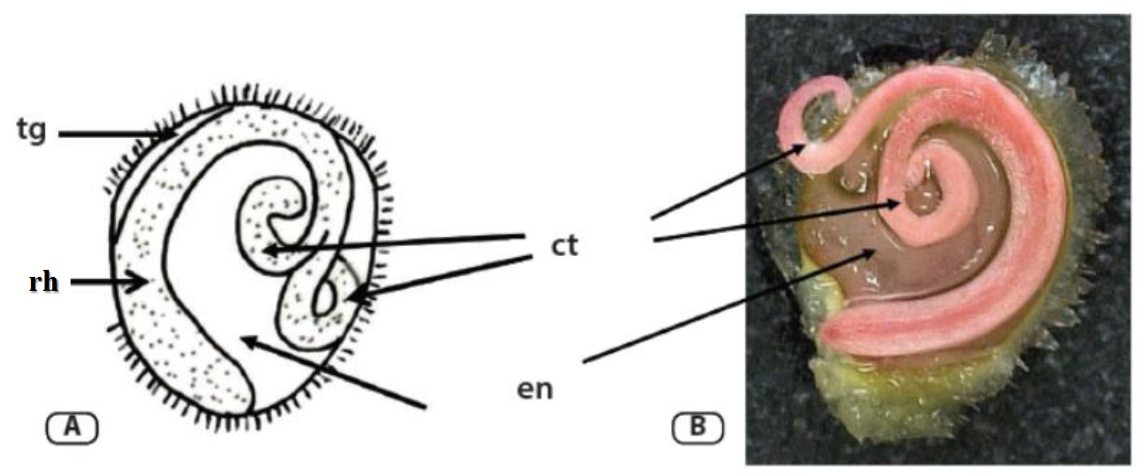
The primary objective of the tetrazolium test (TZT) is to distinguish viable seeds from non-viable ones. This distinction is based on staining patterns and tissue integrity, allowing for a clear separation between the two groups. Moreover, it is critical that the technician has a thorough understanding of seed structures, which may vary depending on the species.
A. Embryo structure of tomato/bell pepper seed; B. embryo stained by the tetrazolium solution. (tg) tegument/seed coat; (ct) cotyledons; (en) endosperm; (rh) radicle-hypocotyl axis. Source: França-Neto, J. B.
In color interpretation, faint normal red typically indicates vigorous tissue, intense red signifies deteriorating tissue, and absence of staining denotes dead tissue. Viable seeds are those capable of producing normal seedlings in a germination test under favorable conditions, once dormancy has been overcome. These embryos typically exhibit uniform staining; however, partially stained embryos may also be considered viable if their staining patterns indicate metabolic activity. Necrotic tissues may be present in different regions of these embryos, and the viability classification depends on the size and position of such areas—not necessarily on the intensity of the staining. Tissue firmness is another crucial criterion that must accompany staining patterns to ensure proper identification and classification of viable seeds.
Non-viable seeds, by contrast, do not meet the above criteria. They often display irregular or poorly defined staining and contain flaccid or unstained essential structures. Seeds exhibiting abnormal embryo development—or other defective vital structures—are considered non-viable, regardless of their staining. Empty seeds must also be classified as non-viable. In the case of conifers, seeds with rudimentary embryos are likewise regarded as non-viable.
Another application of the TZT is to assist in categorizing the vigor levels of different seed lots and in estimating their performance under both optimal and stressful field conditions. Vigorous seeds are capable of developing into normal seedlings, whereas non-vigorous seeds are not. The TZT can also be used in conjunction with germination tests to account for potential dormancy-related discrepancies.
Tomato seeds stained by tetrazolium solution. A, viable and vigorous seed; B, viable non vigorous; C, non-viable seed; D, non-viable (dead) seed. Source: França-Neto, J. B.
In some forest seed laboratories in Brazil, such as the Laboratório de Sementes e Mudas (LASEM) at the Federal University of São Carlos, Sorocaba campus, standardized evaluation forms are used to support the assessment of seed viability and vigor. However, interpretation can still be subjective, as the accuracy of the test relies heavily on the technician’s skill and experience. Expanding the use of the test to a broader range of native species from the Brazilian flora would be extremely beneficial—especially in ecological restoration projects, where high seed diversity and time-sensitive decision-making are common. This highlights the importance of developing and publishing more species-specific TZT protocols, since morphological characteristics, dormancy-breaking requirements, and other factors play a critical role in achieving accurate evaluations.
In forest restoration projects, the tetrazolium test has proven to be a promising tool for evaluating the physiological quality of forest seeds due to its low operational cost and faster results when compared to germination tests. It enables a quick assessment of seed viability, especially for species that require long periods to complete germination, thus supporting timely decision-making by restoration teams—particularly in projects that employ the muvuca technique (see 'Muvuca' Direct Seeding Restoration Method for Biodiversity and People).
Given the test’s practical importance and interpretative challenges, technological solutions are increasingly relevant. With advances in computerized image analysis techniques, efforts should be directed toward developing software tools capable of digitally estimating seed viability and vigor, enhancing the accuracy and objectivity of TZT evaluations.
References
Association of Official Seed Analysts (AOSA). 2010. Seed vigor testing handbook. East Lansing, MI: AOSA.
Brasil. Ministério da Agricultura, Pecuária e Abastecimento. 2025. Teste de Tetrazólio. In Regras para análise de sementes (Chapter 5). Brasília, DF: Secretaria de Defesa Agropecuária. https://wikisda.agricultura.gov.br/pt-br/Laborat%C3%B3rios/Metodologia/Sementes/RAS_TZ
França-Neto, J. B., Krzyzanowski, F. C., & Costa, N. P. 1998. O teste de tetrazólio em sementes de soja. Londrina: EMBRAPA-CNPSo. https://www.infoteca.cnptia.embrapa.br/infoteca/handle/doc/461306
França-Neto, J. B. 1999. Testes de tetrazólio para determinação do vigor de sementes. In F. C. Krzyzanowski, R. D. Vieira, & J. B. França Neto, Vigor de sementes: conceitos e testes. Londrina ABRATES. https://loja.abrates.org.br/vigor-de-sementes
França-Neto, J. B., & Krzyzanowski, F. C. 2018. Metodologia do teste de tetrazólio em sementes de soja. Londrina, PR: Embrapa Soja, Ministério da Agricultura, Pecuária e Abastecimento. (Documentos/Embrapa Soja, ISSN 2176-2937; n.º406).
https://www.infoteca.cnptia.embrapa.br/infoteca/bitstream/doc/1098452/1/Doc406OL.pdf
International Seed Testing Association (ISTA). 2025. International rules for seed testing (Ed. 2025, Chapter 6: The tetrazolium test). Wallisellen, Switzerland: ISTA.