Sponsored by SER’s International Network for Seed-based Restoration
SER2025 field tour group at the National Laboratory for Genetic Resources Preservation. Photo: B.C. Pal
Seventeen delegates to the SER2025 Conference traveled to Fort Collins, Colorado, USA, to visit the National Laboratory for Genetic Resources Preservation (NLGRP), maintained by the US Department of Agriculture’s Agricultural Research Service. The laboratory is the central long-term backup-storage facility for the National Genetics Resources System, a network of more than 20 genebank facilities located nationwide that safeguard collections of seeds and clonal plant material, microbial cultures, and animal samples. The mission of this system is to preserve the genetic diversity of critical genetic resources and make them available for research and use. Sample data is tracked through the Germplasm Resources Information Network (GRIN).
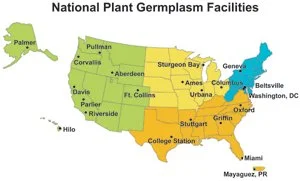
Within the National Genetics Resource System, the National Plant Germplasm System (NPGS) (Fig. 1) stores one of the world’s largest collections of plant genetic resources with close to a million seed samples and more than 12,000 clonal plant accessions. Established to support U.S. agriculture by conserving plant diversity, facilities of the NPGS acquire, evaluate, study, preserve, and distribute agricultural materials crop wild relatives, and other native species. In addition to seeds, collections include pollen, spores, shoot tips, tissue cultures, and dormant buds. Samples may also be grown out at satellite locations to measure plant traits or to provide seed or vegetative material increase.
The NLGRP provides long-term storage for the system’s base collection – backup samples from the collections of the network facilities. It also partners with other government agencies, universities, seed technology companies, and international collaborators to provide backup storage and to conduct cooperative research. Backup of material unique to the United States is stored the Svalbard Genebank in Norway.
Figure 1. The National Plant Germplasm Program includes a network of more than 20 facilities scattered across the United States. Map: USDA ARS.
Seed and Plant Sample Acquisition, Curation, and Management
Plant samples are delivered to the NLGRP from ARS satellite genebanks and other public and private partners (Fig. 2). In addition, exploration trips are supported to fill collection gaps. Samples include Plant Variety voucher specimens, crop wild relative samples, and other native germplasms. Collections are made following protocols for obtaining permits and permissions, plant material harvesting, collection management, and record keeping.
Seed samples received at the NLGRP are equilibrated for several weeks at 23% relative humidity at 5 oC until they reach the moisture content for storage. Collection data in GRIN is checked and samples are assigned accession numbers and labeled. Seeds are carefully cleaned to remove inert material, empty seeds, and contaminants. Samples are weighed and the number of filled seeds determined.
Figure 2. Dr. Hannah Tetreault explaining NLGRP seed acquisition and evaluation. Photo: B.C. Pal.
Seed Banking Native Species for Conservation and Restoration – Crop Wild Relatives and the Seeds of Success Program
Collection of crop wild relatives is a major goal of seedbanks globally. These species contain the genetic diversity needed to meet agricultural challenges now and into the future. They can provide genes needed to improve crop production and quality, increase resilience to environmental stresses, and provide resistance to pests and diseases. Some also have potential medical, horticultural, or industrial uses.
Seeds of Success, a federal native seed collection program, collaborates with non-federal organizations to support a network of interns who harvest samples of native seed for long-term conservation and use in ecological restoration. Peggy Olwell explained that the program was originated by the USDI Bureau of Land Management in 2000, initially in collaboration with the Royal Botanic Garden at Kew, UK, and has provided for collection of more than 23,000 accessions of nearly 5,000 native species from across the United States (Fig. 3). Collections are cleaned, tested, and deposited in the National System for Germplasm Conservation to protect the diversity of our native flora for the future. Samples of each accession are available for research within the NPGS and for use by other researchers and plant material developers. Excess seed is available to improve the quantity, quality, and diversity of plant materials available to meet ecological restoration goals. In addition, many of these species add to the reservoir of crop wild relatives at the NLGRP, while others have received little study for other potential uses.
Figure 3. Seeds of Success has collected seed of native species across the United States. Map: USDI Bureau of Land Management.
Seed Testing and Germination Research
Viability of all seed lots is tested prior to storage to determine the percentage of seeds that are alive and capable of producing normal seedlings. Samples in storage are retested at specified times to check for changes in viability. Dr. Shaimaa Ibrahim (Fig. 4) explained that analysts use protocols available in Association of Official Seed Analyst literature, the Seed Information Database, and other sources. The number of seeds tested is dependent on the size of the sample. All samples scheduled for cryopreservation storage in liquid nitrogen have paired tests performed to check for damage caused by the liquid nitrogen.
Protocols are lacking for determining the viability of many wild-collected species, thus protocols for related species are employed or modified for testing. Researchers are working to develop and test protocols for additional native species, an often-difficult task due to high intraspecific variability or complex dormancy.
Figure 4. Dr. Shaimaa Ibrahim describing seed quality assessment procedures. Photo: S. Swim.
Clonal Sample Storage and Research
The National Plant Germplasm System stores clonal material of plants that (1) reproduce vegetatively and produce no or few seeds, (2) do not survive in cold storage, (3) are difficult to propagate from seed, (3) for which seed storage protocols are not available, or (4) when it is necessary to maintain a specific genotype asexually as is the case for some cultivars. These plants may be maintained as live plants, but backup material is maintained by the NLGRP and elsewhere through cryopreservation in liquid nitrogen. Woody plants such as apples and other cold-hardy trees and shrubs can be stored using dormant leaf buds. Herbaceous species and some tropical trees are stored using shoot tips.
Remi Bonnart, a plant cryopreservation technician in the Clonal Research Unit, demonstrated techniques for preparing materials for cryopreservation (Fig. 5). Dormant buds are dried at low temperatures to reduce water content, sealed in plastic tubes, and dried to -30 oC. They are then placed in liquid nitrogen for long- term storage. Shoot tips (1-2 mm long) are taken from plants grown in tissue culture or from plants grown in the field or greenhouse and conditioned to increase their tolerance of dehydration and freezing. They are then given treatments to reduce water content. Prepared shoot tips are cooled rapidly in liquid nitrogen, transferred to vials, and placed in cryrotanks for long-term storage.
Research at the lab adds to preservation methods for additional species. Progress can be slow as each step must be carefully tested and modified until successful storage and rehydration can be assured.
Figure 5. Remi Bonnart discussing clonal preservation. Photo: N. Shaw.
Figure 6a. Dormant buds and shoot tip generation for cryopreservation (left). Photo: B. Leger.
Figure 6b. Vials of shoot tips prepared for vitrification in liquid nitrogen (right). Photo: B. Leger.
Storage
Documented, cleaned, evaluated, and prepared seeds and clonal materials are moved to storage in the vault, a separate part of the NLGRP facility with a capacity of 1.5 million samples. The vault is constructed to withstand disasters and equipped with backup electrical generators to maintain storage conditions in case of an emergency. Seeds are stored in conventional cold storage at -18°C or in liquid nitrogen at -196°C for cryogenic storage. Appropriate storage conditions for each seed sample are determined by consideration of the seed type, size, quality, sample size, and other factors.
The conventional storage area is heavily insulated and filled with movable shelving racks that maximize storage space (Fig. 8). Seeds are stored in heat-sealed, moisture-proof, foil-laminated bags with barcoding to indicate their identification and location. Seedlots in conventional storage may remain viable for up to 50 years, but their viability is checked periodically due to the high variability within species and seedlots.
The cryogenic vault area is filled with heavily insulated cryotanks (Fig. 8) that are filled with liquid nitrogen weekly. Samples are prepared for cryogenic storage by placing them in clear, barcoded tubes that are closed, placed in labeled metal boxes, and stored on racks above the nitrogen in the tanks (Fig. 9). Oxygen content of the air in the cryogenic vault is monitored continuously, and nitrogen is shut off in emergency situations.
Figure 7. Amy Gurza describing procedures for managing seedlots in cold storage. Photo: S. Frischie
The cryogenic vault area is filled with heavily insulated cryotanks (Fig. 8) that are filled with liquid nitrogen weekly. Samples are prepared for cryogenic storage by placing them in clear, barcoded tubes that are closed, placed in labeled metal boxes, and stored on racks above the nitrogen in the tanks (Fig. 9). Oxygen content of the air in the cryogenic vault is monitored continuously, and nitrogen is shut off in emergency situations.
Figure 8. Amy Gurza describing cryotank storage. Photo: S. Frischie
Figure 9. Hannah Tetreault and Jennifer Kendall with a demonstration cryotank. Photo: S. Swim
Seed Biology and Genetics Research
At the NLGRP, seed research focuses on methods for more efficiently managing, evaluating, storing, and using seed of the many thousands of seed accessions at the laboratory. Results contribute not only to improved seed banking but also to reducing costs, labor, and improving the potential for expanding the seed bank.
Dr. Christina Walters (Fig. 10) conducts seed physiology and biophysics research aimed at understanding the mechanisms of seed aging to aid in detecting early signs of deterioration. Her results contribute to formulation of more rapid and non-destructive techniques for monitoring seed quality and effective long-term storage practices to slow the aging of diverse seed lots, thus potentially reducing the frequency of grow-outs to maintain accessions.
Dr. Walters research aims to understand changes in seed metabolism and cytoplasm structure during drying and in cryostorage to monitor the rate of deterioration and to determine when viability has dropped to the point that the accession must be replaced. Approaches for monitoring include measuring the production of organic compounds during lipid breakdown (Figure 11). Another approach involves assessment of the rate of RNA fragmentation under low temperature storage as a means of monitoring the rate of viability loss.
Figure 10. Dr. Christina Walters (left) discussing seed storage physiology. Photo: N. Shaw
Figure 11. Volatiles emitted by aging seeds are collected for spectroscopy analysis. Photo: S. Swim
Dr. Chris Richards, a population geneticist and lead scientist at the lab, described the core functions of the lab as (1) collect it, (2) keep it alive, (3) don’t change it, and (4) make it available. Dr. Richards uses genomics data to analyze population structure and genetic diversity across the geographic range of individual species. This data can be used to aid to protect accession identity. It also aids in identifying areas for further collection to ensure that the diversity of a species has been captured and to focus collection efforts on areas that are likely to have alleles useful for agriculture and for meeting climate change and other challenges (Fig. 12).
Figure 12. Display of diverse seeds. Photo: S. Frishie.